What is Clinical Trial Software
Kohezion's Clinical Trial Management Software is your all-encompassing solution for managing the complex processes of clinical trials. From patient recruitment and enrollment to data collection and analysis, this software simplifies each step. It facilitates seamless collaboration between researchers, sponsors, and regulatory bodies, ensuring compliance and efficiency. This Clinical Trial Software speeds of clinical research, making it an indispensable tool for any clinical trial operation.
Clinical Trial Software Features
How to use Clinical Trial Software
Trial Planning and Design
Participant Recruitment and Enrollment
Site Management and Monitoring
Data Collection and Electronic Data Capture (EDC)
Regulatory Compliance and Document Management
Budgeting and Financial Management
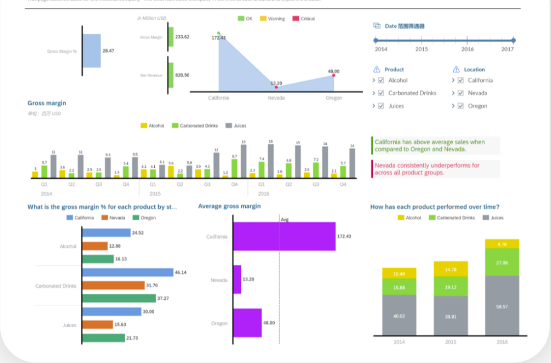
Study Analytics and Reporting
Collaboration Tools
Inventory and Supply Management
Clinical Trial Software is Designed For:
Related Healthcare Software
What is Next?
- Get help building your app: Request a demo and we'll install this application to your trial Kohezion account.






Frequently Asked Questions
Kohezion's Clinical Trial Management Software is your all-encompassing solution for managing the complex processes of clinical trials. From patient recruitment and enrollment to data collection and analysis, this software simplifies each step. It facilitates seamless collaboration between researchers, sponsors, and regulatory bodies, ensuring compliance and efficiency. This Clinical Trial Software speeds of clinical research, making it an indispensable tool for any clinical trial operation.
- Trial Planning and Design
- Facilitate the planning and design of clinical trials, including protocol development, in the 'Trial Planning' module. Streamline trial setup processes to align with research objectives.
- Participant Recruitment and Enrollment
- Manage participant recruitment, screening, and enrollment processes in the 'Participant Management' section. Efficiently track participant progress throughout the trial.
- Site Management and Monitoring
- Oversee clinical trial sites and conduct monitoring activities in the 'Site Management' module. Ensure compliance with trial protocols and regulatory standards.
- Data Collection and Electronic Data Capture (EDC)
- Collect clinical trial data using electronic data capture systems in the 'Data Collection' section. Ensure data accuracy and integrity.
- Regulatory Compliance and Document Management
- Manage regulatory compliance and trial documentation, including the Trial Master File (TMF), in the 'Compliance' module. Streamline submissions to regulatory bodies.
- Budgeting and Financial Management
- Control trial budgets, financial planning, and expense tracking in the 'Financial Management' section. Ensure efficient financial oversight of clinical trials.
- Study Analytics and Reporting
- Analyze trial data and generate reports on study progress, enrollment, and outcomes in the 'Analytics' module. Utilize data for strategic decision-making.
- Collaboration Tools
- Enhance team collaboration and communication among researchers, sponsors, and CROs using integrated tools in the 'Collaboration' section.
- Inventory and Supply Management
- Manage trial supplies, including drugs and equipment, efficiently in the 'Inventory Management' module. Ensure adequate supply throughout the trial.